How To Convert Grams To Moles
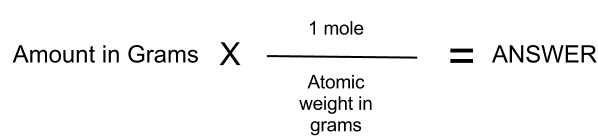
How To Convert Grams To Moles - Divide the number of grams of the substance by the molecular mass The answer will be the number of moles of the compound See an example of converting grams to moles Converting grams to moles is easy with these few steps Soon you will have the mass of your substance The simplest type of manipulation using molar mass as a conversion factor is a mole gram conversion or its reverse a gram mole conversion We also established that 1 mol of Al has a mass of 26 98 g Example PageIndex 1
How To Convert Grams To Moles
How To Convert Grams To Moles
The number of moles you have of a compound can be calculated by dividing the number of grams of the compound by the molecular mass of the compound. The formula looks like this: moles = grams of compound/molar mass of compound. 2. Plug your numbers into the formula. Calculating the mass of a sample from the number of moles it contains is quite simple. We use the molar mass (mass of one mole) of the substance to convert between mass and moles. When writing calculations, we denote the molar mass of a substance by an upper case “M” (e.g. M (Ne) means “the molar mass of neon”).
6 2 Gram Mole Conversions Chemistry LibreTexts

How To Convert Cups To Grams Formula Convert Cups To Grams Uk How
How To Convert Grams To MolesAll you need to do is correctly enter your formula, choose whether you want a conversion from grams to moles or a conversion from moles to grams, and, in case of g to mol, enter the mass, or, in case of mol to g, enter the moles. Grams to moles example problem. Problem: Convert 25.0 grams of KMnO4 to moles. Solution: Solution First look up the atomic masses for carbon and oxygen from the periodic table The atomic mass of C is 12 01 and the atomic mass of O is 16 00 The formula mass of CO 2 is 12 01 2 16 00 44 01 Thus one mole of CO 2 weighs 44 01 grams This relation provides a conversion factor to go from grams to moles
Answer link. To convert from grams to moles, you must divide by the molar mass of the substance. For example, water has a molar mass of 18 g/mol. Therefore, a 25 g sample of water contains (25 g)/ (18 g/ (mol)) = 1.39 mol of water molecules. How To Convert Grams To Moles 8 Steps with Pictures WikiHow As My Father Always Said when In Doubt Convert To Moles How To
6 3 Converting Between Grams And Moles Chemistry LibreTexts
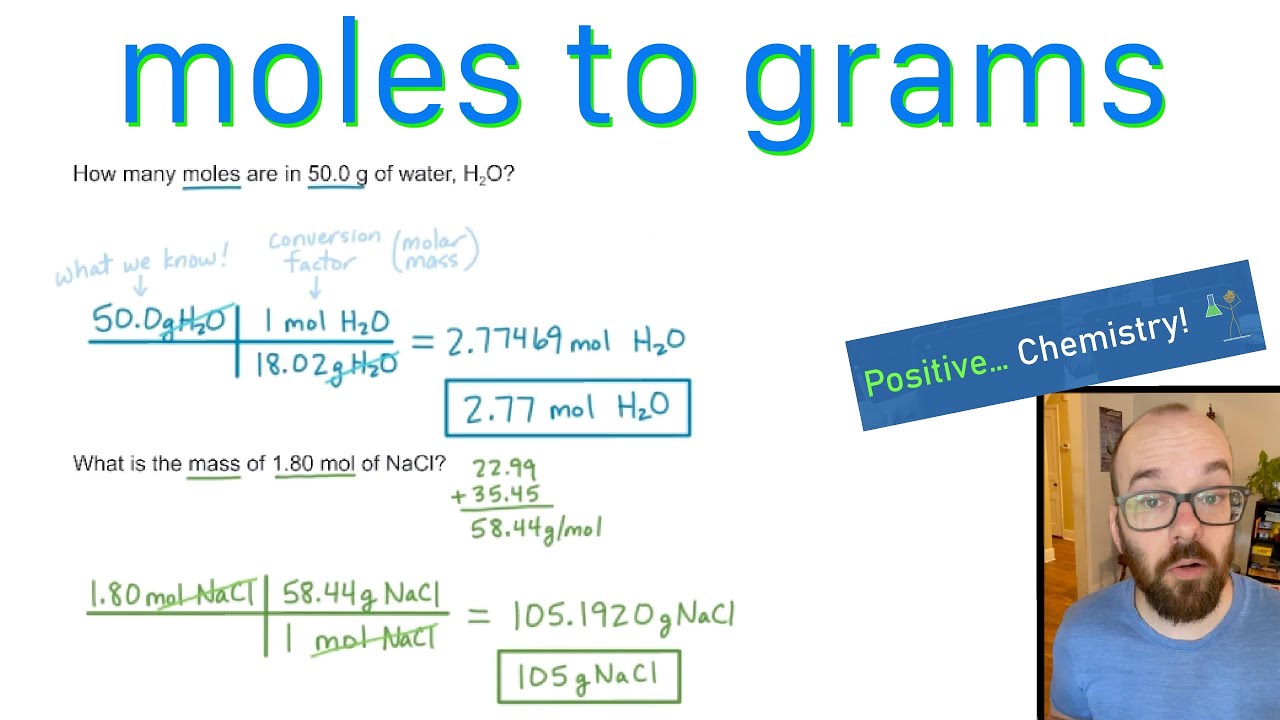
Moles To Grams How To Convert Positive Chemistry YouTube
To convert between grams and moles, we need to use the molar mass. The molar mass is the mass of one mole of an element and is found on the periodic table. If we look this number up for helium, we find that helium has a molar mass of 4.0 grams per mole. So one mole of helium has a mass of 4.0 grams. We can use the molar mass as a. ShowMe Grams
To convert between grams and moles, we need to use the molar mass. The molar mass is the mass of one mole of an element and is found on the periodic table. If we look this number up for helium, we find that helium has a molar mass of 4.0 grams per mole. So one mole of helium has a mass of 4.0 grams. We can use the molar mass as a. Moles Converting Between Grams And Particles YouTube Converting Grams To Moles Formula Calculation Examples Video

Grams To Moles And Back YouTube

How To Convert Grams To Grams Stoichiometry Examples Practice Problems

C mo Convertir Gramos A Moles 8 Pasos con Fotos

Moles To Grams Conversion Examples

How To Convert Between Molecules Moles And Grams Examples Practice

Converting Grams To Moles YouTube
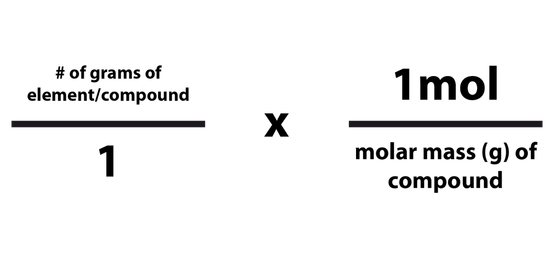
Converting Grams And Moles The Mole And Its Applications Free
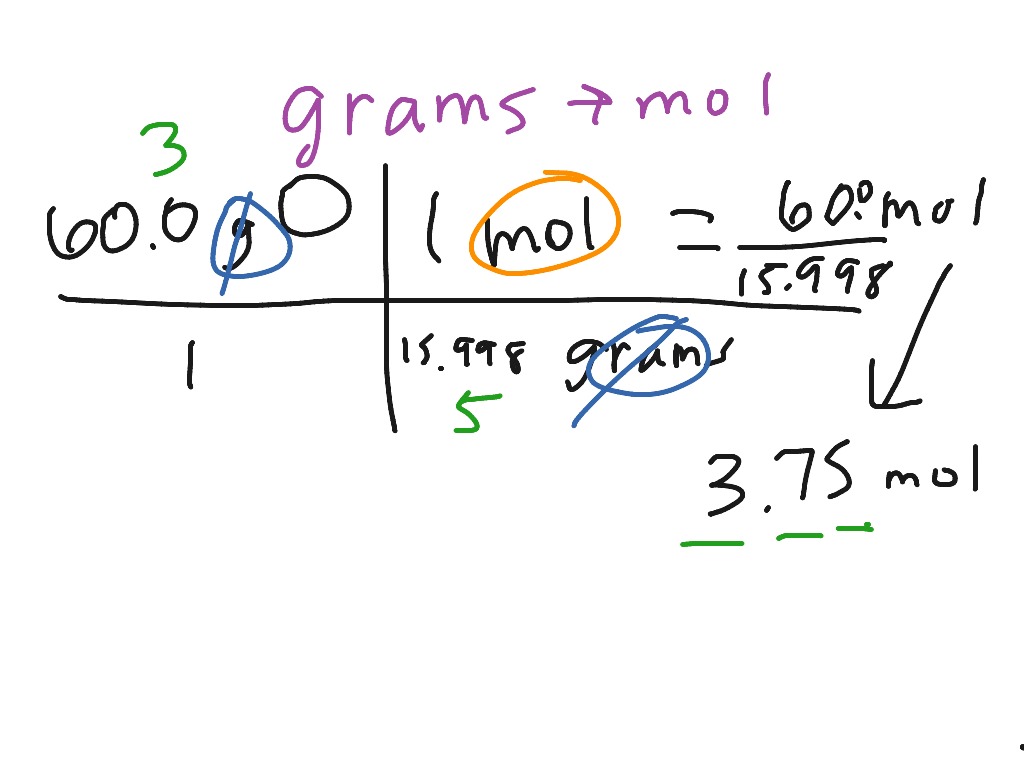
ShowMe Grams

How To Convert Grams To Moles Grams To Moles Weighing Scale Lab
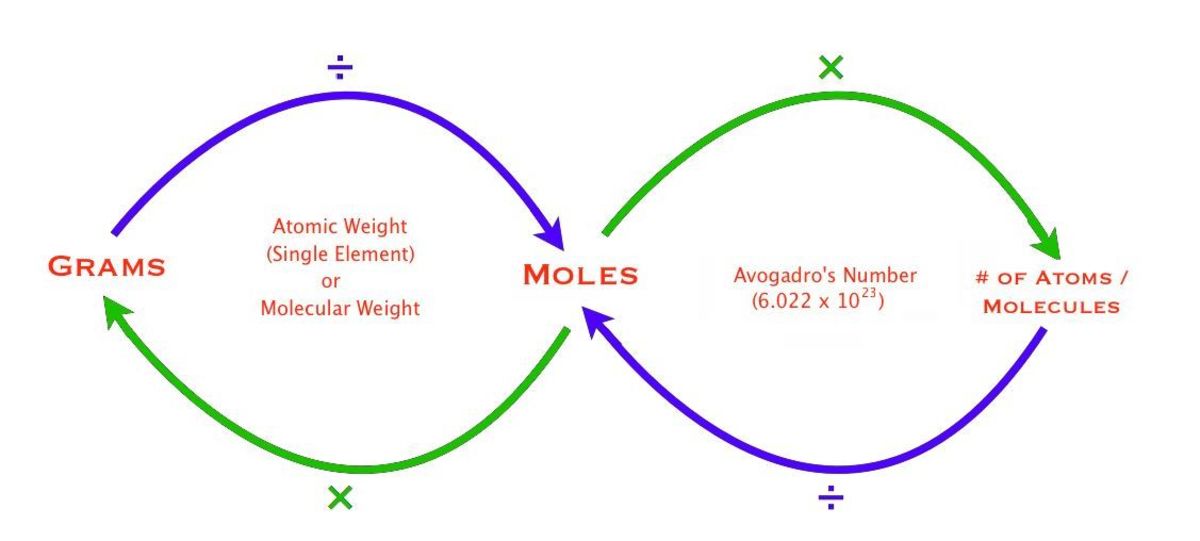
Grams To Moles Conversion Chart