How To Find The Mass Of An Atom
How To Find The Mass Of An Atom - The mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted average mass of all the isotopes present in a naturally occurring sample of that element This is equal to the sum of each individual isotope s mass multiplied by its fractional abundance The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom components protons neutrons and electrons or vice versa In addition you can define the charge of ions with known numbers of protons and electrons This article will provide you with the following Definitions of Atom
How To Find The Mass Of An Atom
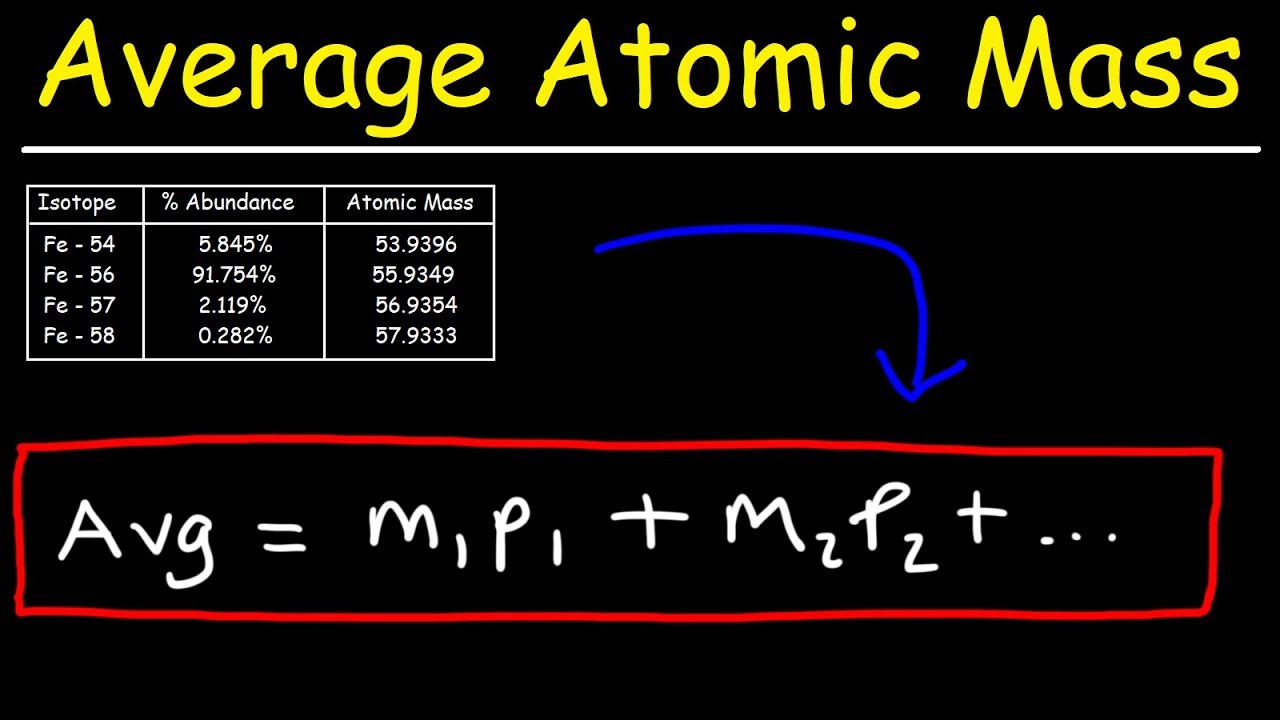
How To Find The Mass Of An Atom
To find a reasonable approximation of the atomic mass of an atom in atomic mass units, we can simply add the number of protons and neutrons. So the atomic mass formula is: atomic mass (u) ≈ number of protons + number of neutrons. Or. A = Z + N A =Z +N. where: A A — Mass number (total number of protons and neutrons); To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons. Example: Find the atomic mass of an isotope of carbon that has 7 neutrons. You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.
Atom Calculator
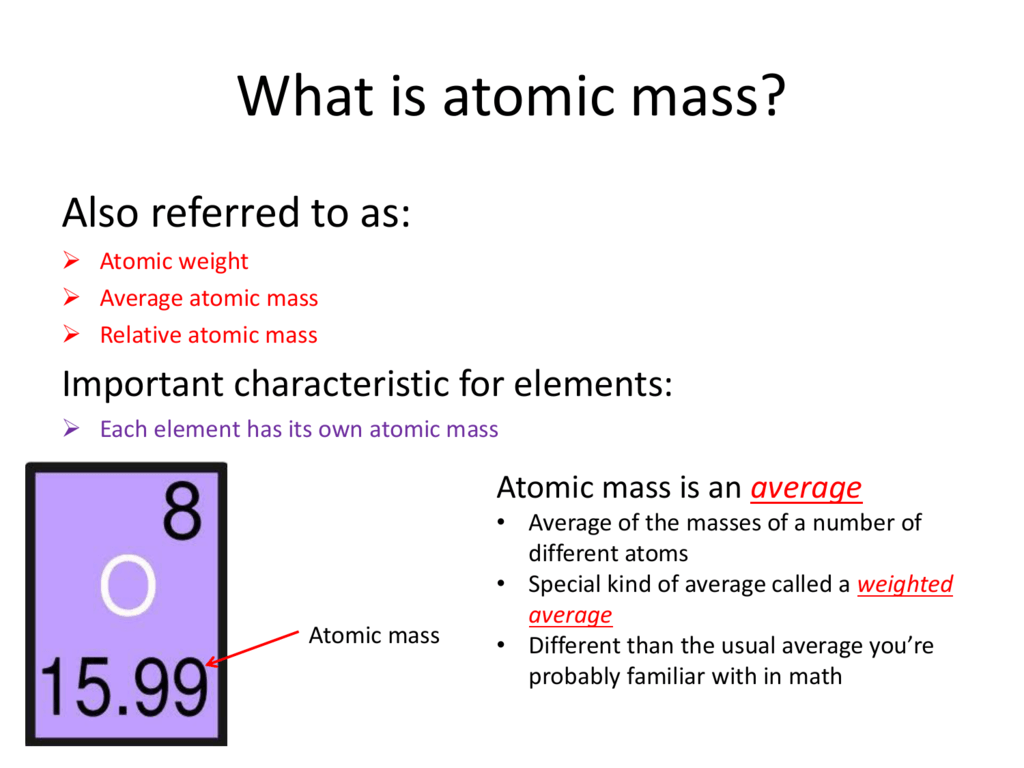
What Is Atomic Mass
How To Find The Mass Of An AtomIf the mass of a single atom is needed, simply count the number of protons and neutrons in the atom. This is more properly called the mass number, to distinguish it from standard atomic mass. Example: What is the atomic mass of a carbon isotope with 8 neutrons? Carbon is element 6. Step 1 Atomic mass of each isotope x Abundance 100 34 96885 0 7578 26 50 i 36 96590 0 2422 8 95 ii Step 2 Adding i and ii the atomic mass of the given sample is determined 26 50 8 95 35 45 Thus the atomic mass of the given sample of chlorine was found to be 35 45
How to calculate atomic weight from atomic mass and percent abundance of carbon isotopes. Created by Sal Khan. Questions. Tips & Thanks. Want to join the conversation? Sort by: Top Voted. Valentin Sánchez Ozuna. 7 years ago. If Carbon-12 has an atomic mass of 12 amu, why does Carbon-13 have 13.0034 amu? Why isn't 13 amu? •. ( 77. How To Find The Number Of Neutrons In An Element Redesigngreece 3 04 Molar Mass
How To Calculate Atomic Mass ThoughtCo

How To Calculate The Atomic Mass Of An Isotope Average Atomic Mass
The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. This value is the average atomic mass of the element because elements may have more than one naturally occurring isotope. Periodic Table Element With Atomic Mass And Atomic Number Dynamic
The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. This value is the average atomic mass of the element because elements may have more than one naturally occurring isotope. Atomic Mass Of Hydrogen Yoga vedanta Atomic Number And Mass Number mov YouTube
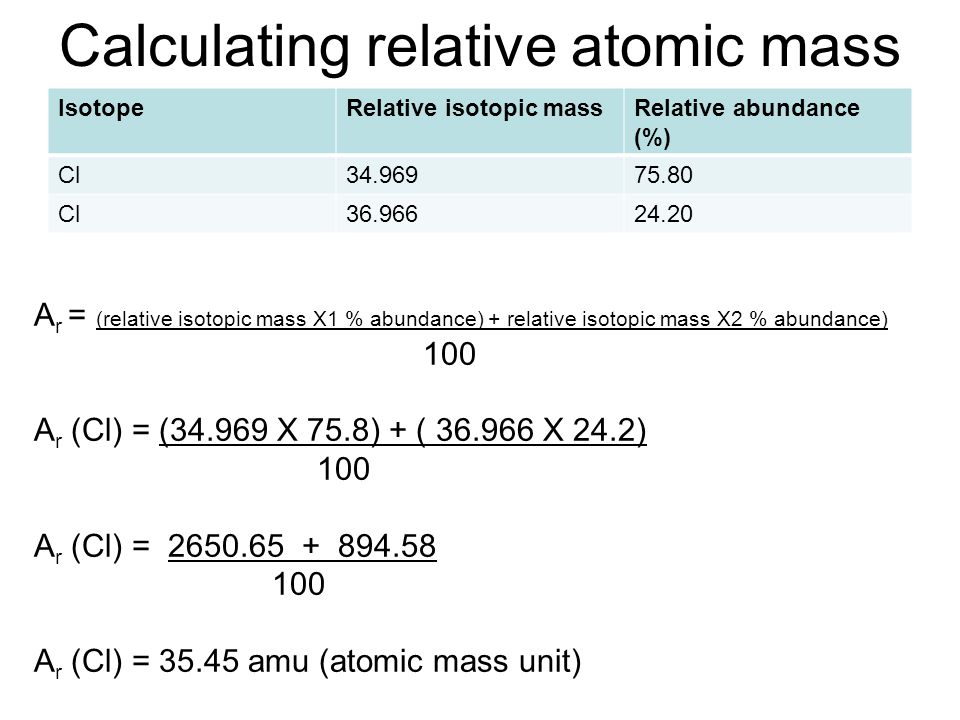
How To Find Atomic Mass And Number Of Elements
Building Ions
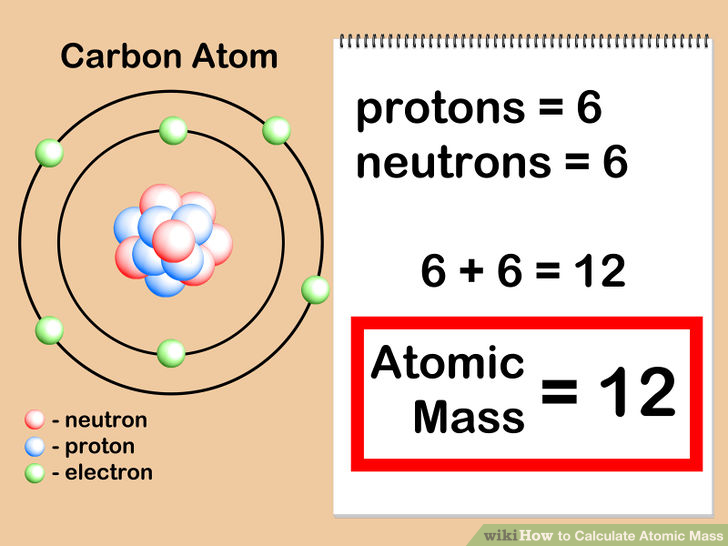
Semester 2 Study On Emaze
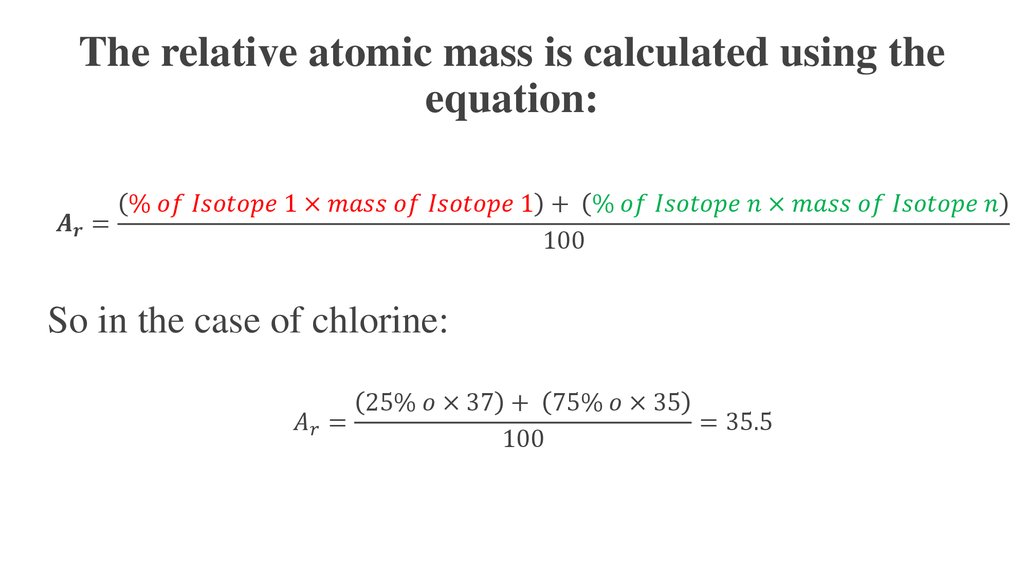
Atomic Mass Online Presentation
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Difference Between Atomic Mass And Mass Number
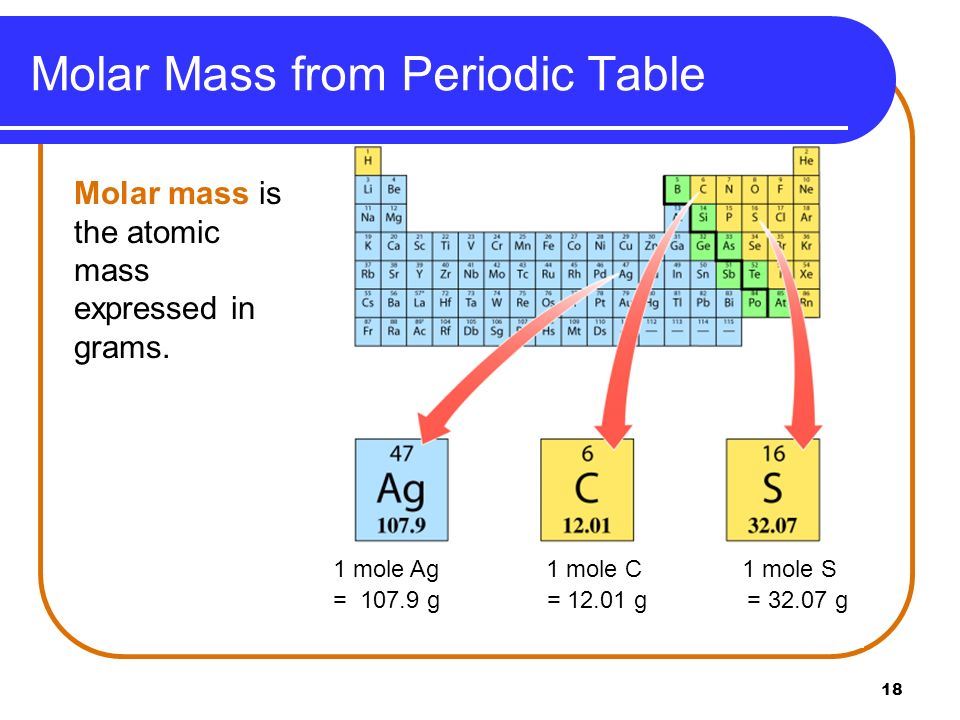
Atomic Mass And Molecular Mass Definition Difference Mass Spectrometry
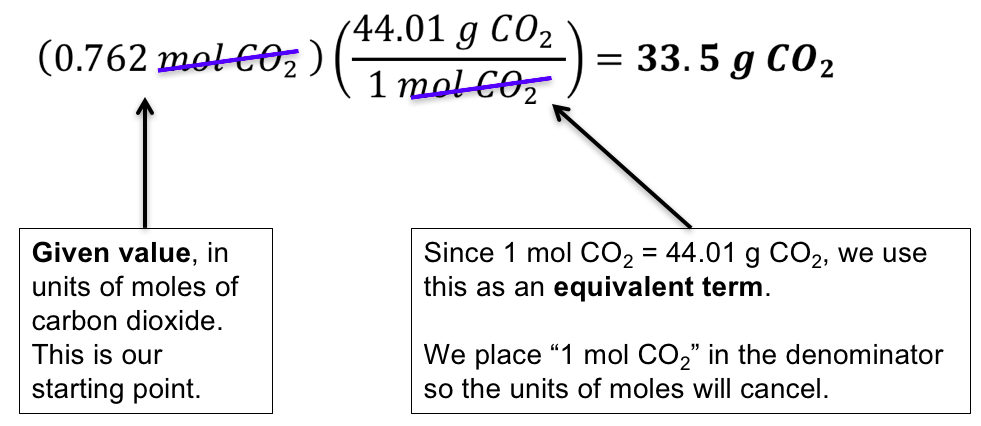
Molar Mass Conversion Chart
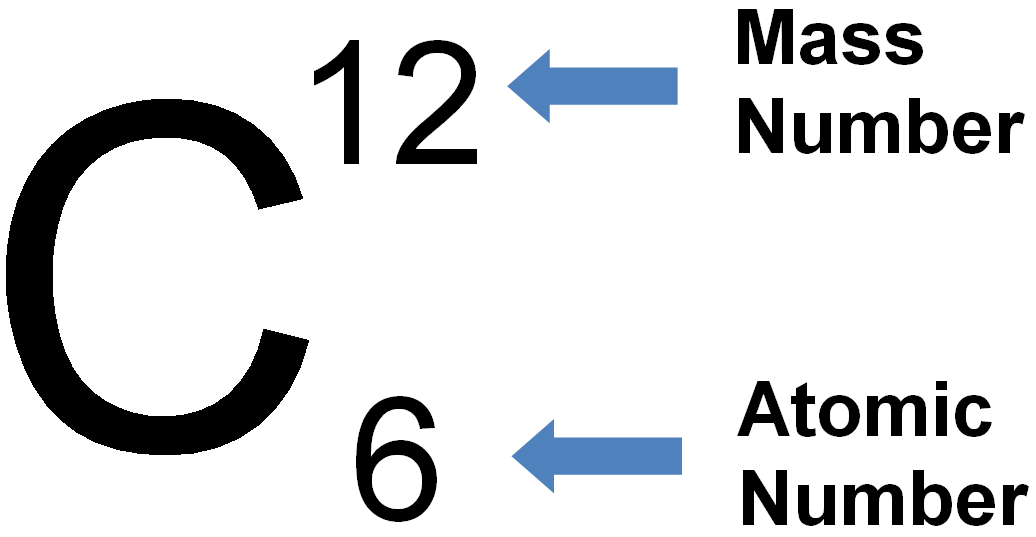
Periodic Table Element With Atomic Mass And Atomic Number Dynamic
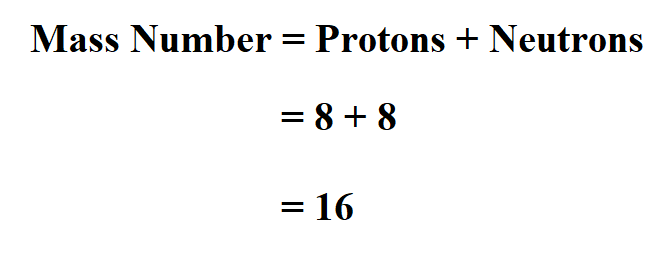
How To Calculate Mass Number
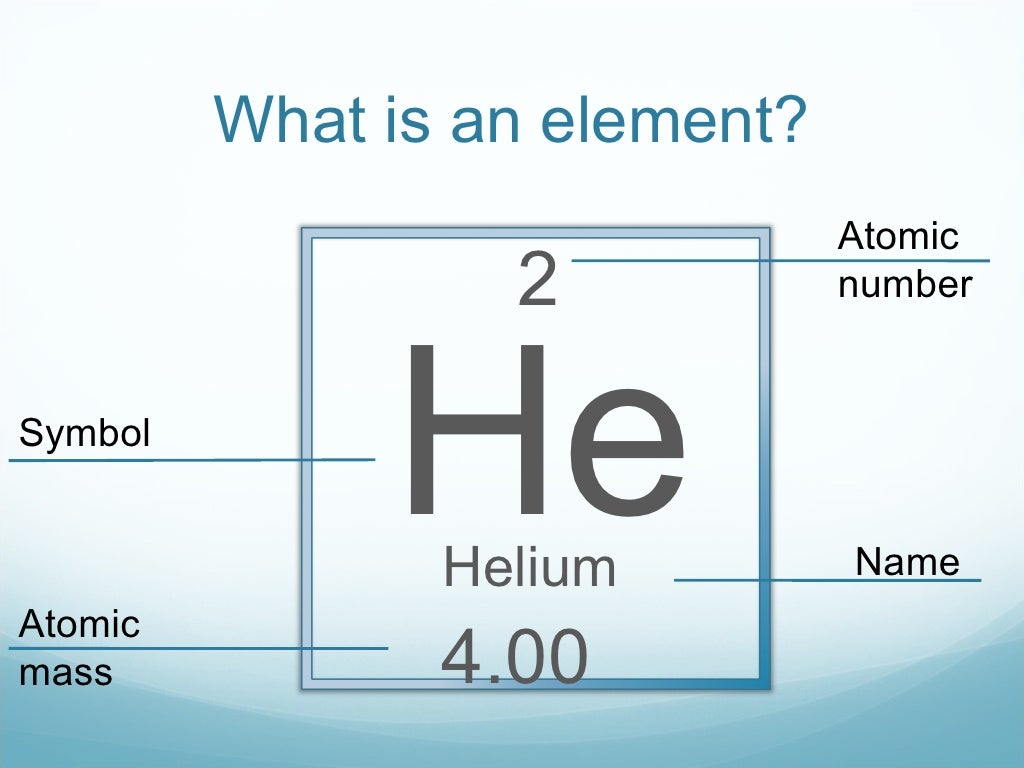
What Is Atomic Mass
.PNG)