Root Mean Square Speed Formula
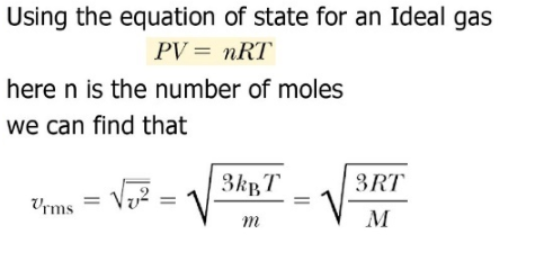
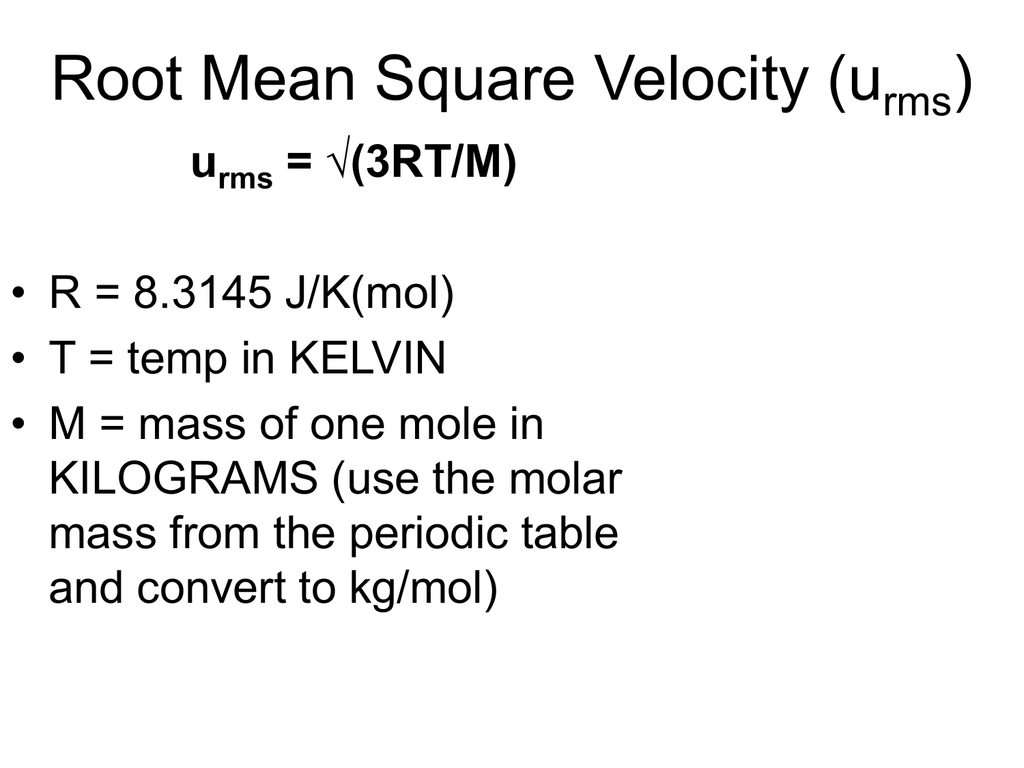
Root Mean Square Speed Formula - Root Mean Square Speed The pressure of an ideal gas equation includes the mean square speed of the particles Where c average speed of the gas particles has the units m2 s 2 Since particles travel in all directions in 3D space and velocity is a vector some particles will have a negative direction and others a positive direction The root mean square velocity RMS velocity is a way to find a single velocity value for the particles The average velocity of gas particles is found using the root mean square velocity formula rms 3RT M rms root mean square velocity in m sec R ideal gas constant 8 3145 kg m 2 sec 2 K mol
Root Mean Square Speed Formula

Root Mean Square Speed Formula
The rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. Doubling this average velocity doubles the number of collisions between gas molecules and the walls of a. Compare formulas for average kinetic energy Ek and kinetic energy of motion of the gas molecules to find the root mean square velocity equation: \small \begin {split} E_ {\rm k} &= \frac {3} {2}RT\\ [1em] E_ {\rm k} &= \frac {1} {2}m\upsilon^ {2}\\ [1em] \upsilon_ {\rm rms}&=\sqrt {\frac {3RT} {M}} \end {split} E k E k υrms = 23RT = 21mυ2 = M 3RT.
Calculate Root Mean Square Velocity Of Gas Particles

CHEMISTRY 101 Root Mean Square Velocity Of Gas Molecules YouTube
Root Mean Square Speed Formula2.5K. 298K views 7 years ago. This chemistry video tutorial focuses on the root mean square velocity equation. It gives an example / practice problem showing you how to calculate the root. We can solve overline K frac 1 2 m overline v 2 frac 3 2 k BT for a typical speed of a molecule in an ideal gas in terms of temperature to determine what is known as the root mean square rms speed of a molecule
So if I want to find the root, mean square speed of core in gas or oxygen gas, this is the formula I would use. So root mean square speed is abbreviated as the R M s equals. Now the term root is used in naming this so route would mean square root, so it equals the square root of three rt over em. Root Mean Square Speed YouTube PPT Lecture 1 Temperature Ideal Gas Ch 1 PowerPoint
Root Mean Square Velocity Calculator

Understanding Kinetic Energy Root Mean Square Speed YouTube
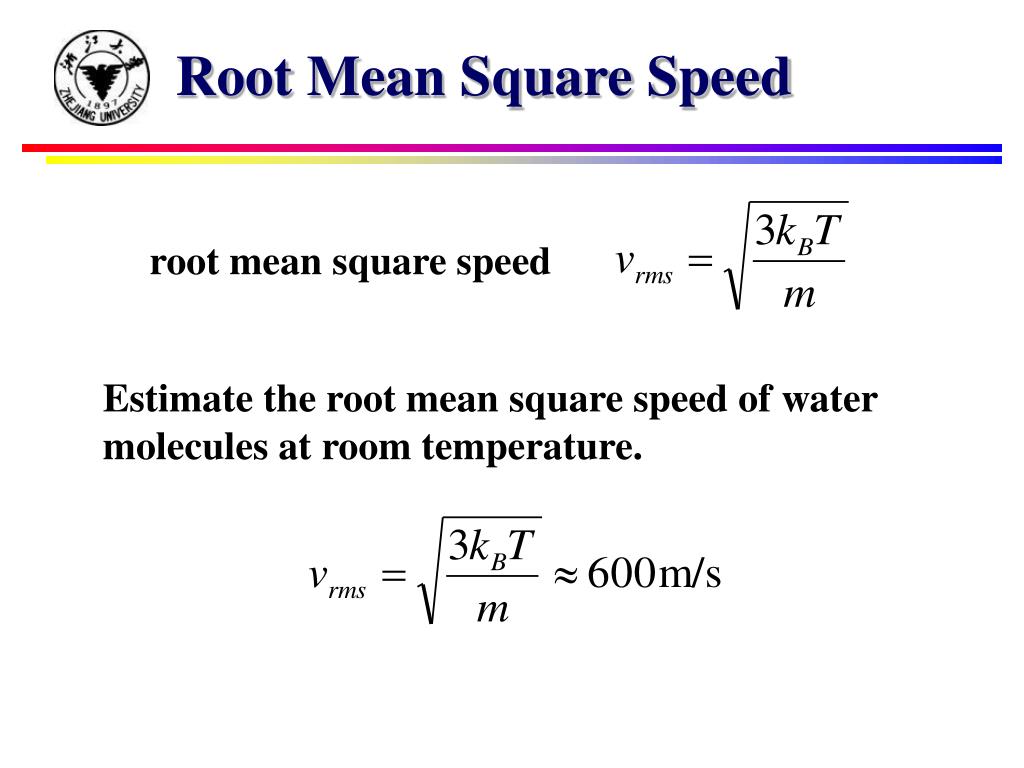
The root-mean-square speed is the measure of the speed of particles in a gas, defined as the square root of the average velocity-squared of the molecules in a gas. It is represented by the equation: v r m s = 3 R T M v_{rms}=\sqrt{\frac{3RT}{M}} v r m s = M 3. PPT Microscopic Model Of Gas PowerPoint Presentation Free Download
The root-mean-square speed is the measure of the speed of particles in a gas, defined as the square root of the average velocity-squared of the molecules in a gas. It is represented by the equation: v r m s = 3 R T M v_{rms}=\sqrt{\frac{3RT}{M}} v r m s = M 3. How To Calculate Root Mean Square Velocity Examples And Practice Pin On MCAT

Chem 101 Calculating Root Mean Square Velocity 2 Youtube Images
Mineral Insanidade Carvalho V Rms Calculation Cura Quartafeira Ver o

Root Mean Square Calculator
Root Mean Square Velocity urms
Fine Beautiful Root Mean Square Velocity Derivation Pdf What Is

Root Mean Square Speed Chemistry Video Clutch Prep
Root Mean Square Speed RMS Vs Average Speed YouTube
PPT Microscopic Model Of Gas PowerPoint Presentation Free Download

Root Mean Square Velocity Slidesharetrick

Root Mean Square Velocity YouTube
