What Are The Physical Properties Of Metals
What Are The Physical Properties Of Metals - Q 1 What are the 5 physical properties of metals Ans The five physical properties of metals include malleability ductility lustre sonority thermal conductivity and density Q 2 Is iodine a metal Ans No iodine is a non metal Q 3 What are the physical and chemical properties of metals The metals are distinguished by their chemical and physical properties such as malleability ductility ionization and bonding properties etc Properties of Metals Examples of metals are gold aluminium iron and
What Are The Physical Properties Of Metals

What Are The Physical Properties Of Metals
The chemical elements can be broadly divided into metals, metalloids, and nonmetals according to their shared physical and chemical properties. All metals have a shiny appearance (at least when freshly polished); are good conductors of heat and electricity; form alloys with other metals; and have at least one basic oxide. Physical Properties of Metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Other properties include: State: Metals are solids at room temperature with the exception of mercury, which is liquid at room temperature (Gallium is.
Properties Of Metals And Nonmetals Physical And Chemical

Physical Properties Of Metals Physical Properties Of Metals They Include Such Things As
What Are The Physical Properties Of MetalsWhen a load is applied to metal, the atomic structure itself is strained, being compressed, warped or extended in the process. The atoms comprising a metal are arranged in a certain geometric pattern, specific for that particular metal or alloy, and are maintained in that pattern by interatomic forces. Chemical properties 1 High molecular weights Metals have a high atomic number and also atomic weights 2 Metallic oxides All metals can form oxides These oxides are alkaline in nature and have high pH above 7 when 3 They react with acids Metals react with acids and get eroded slowly 4
metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Approximately three-quarters of all known chemical elements are metals. The most abundant varieties in the Earth’s crust are aluminum, iron, calcium, sodium, potassium,. Physical Properties Of Matter Worksheet Free Printable PDF For Kids SCIENCE BLOG YEAR 4 THE PROPERTIES OF MINERALS
7 6 Metals Nonmetals And Metalloids Chemistry LibreTexts
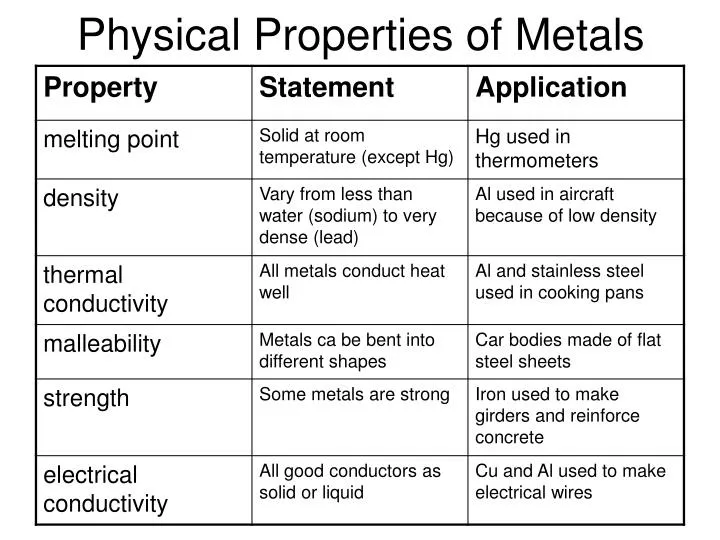
PPT Physical Properties Of Metals PowerPoint Presentation ID 5519685
GCSE AQA Synergy Metals - AQA Synergy Properties of metals Metals have giant structures of atoms with delocalised electrons. This explains their high melting and boiling points and why they. Teacher history ru
GCSE AQA Synergy Metals - AQA Synergy Properties of metals Metals have giant structures of atoms with delocalised electrons. This explains their high melting and boiling points and why they. Chemical Properties Of Iron Metal Chemical Properties Of Metals And Non Metals BYJU S 2022 Four Physical Properties Of Iron Metal What Is The Physical And Chemical Properties Of Iron
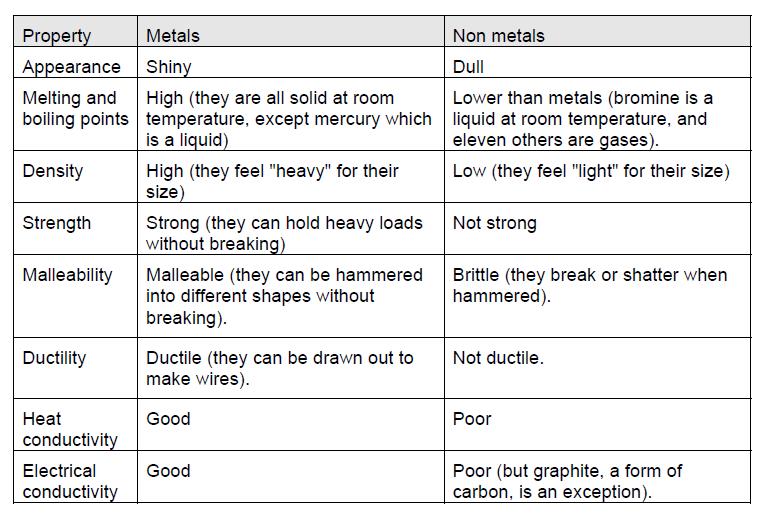
Metals Chapter WGHS Junior Science

What Are The Physical Properties Of Metals Techy Bois
Physical Properties Of Metals And Non Metals Basic Definition Examples Diagrams

PPT CHAPTER 4 MATERIALS METALS AND NON METALS PowerPoint Presentation ID 3878528
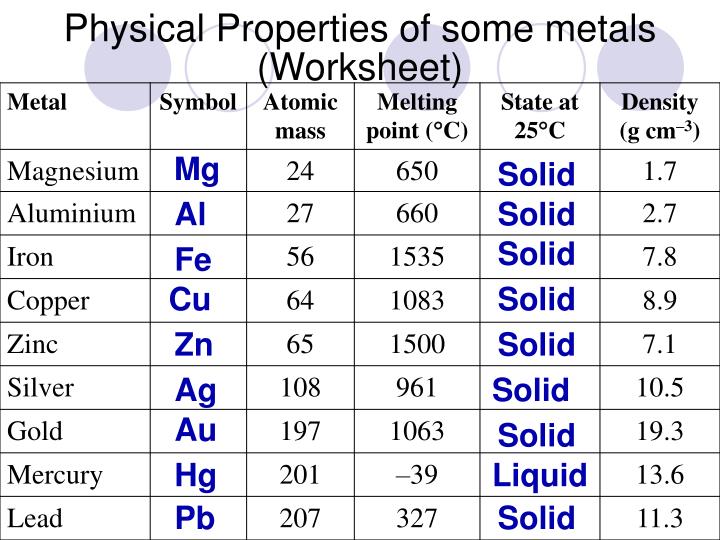
PPT Physical Properties Of Metals And Their Uses PowerPoint Presentation ID 3843269

Question Video Identifying The List Of Physical Properties Used To Classify Elements As Metals

Which Of The Following Is A Characteristic Of Nonmetals LelandkruwCampbell
:max_bytes(150000):strip_icc()/metals-versusnonmetals-608809-v3-5b56348946e0fb0037001987.png)
Teacher history ru

Nickel Archives Selftution

PPT What You Need To Know What Is A Metal What Are The Physical Properties Of Metals
